Molecules and Basic Bonding
This is a 25 minute screencast on bonding that should be entirely review for most of you. Listen to it if you think you would benefit from the review.
Here is a link: screencast covering Bonding Basics.
There is a “table of contents” icon (just 4 horizontal lines at the lower right as you mouse over it). If you click on it, it will list several topics. You can skip to those you want to hear again.
Also, here is a PDF on functional groups that I think you should look over.
Finally, here is a primer on "molecular shorthand" we use when we draw molecules.
Structural Shorthand
When we are writing out a larger molecule, we often use a shorthand notation. So, it is important that you know what they mean. Bonds are line segments, atoms are at the junction or ends of line segments. Double line segments mean double bonds. Beyond that, it’s really simple and based on two rules:
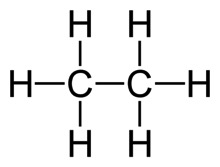
Each end of the line segment is a C, which I don’t write. Each C must have three more hydrogens to fill it out, which I also don’t write.
However, consider this molecule:
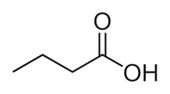 It is a really short fatty acid called “butyric acid.” How many carbons and hydrogens are there.
It is a really short fatty acid called “butyric acid.” How many carbons and hydrogens are there.
I’ll wait.
You should have gotten 4 carbon, 8 hydrogen. Here it is explicitly.
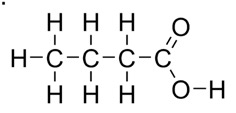
The carbonyl carbon has four bonds already (two to the O on top, one to the O to its right, and one to the carbon on the left) and has no room for hydrogens. Carbons in the middle of a chain have two hydrogens and the one on the end has to have 3.
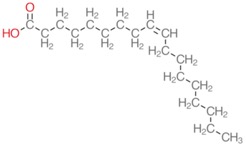
Here’s a much harder one:
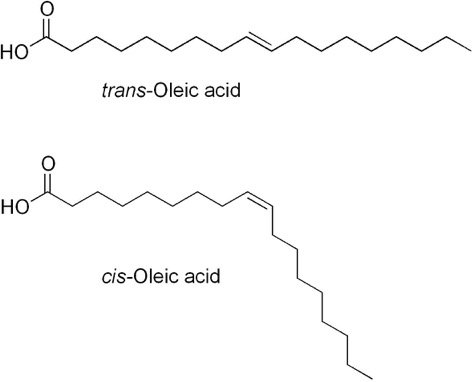
We will talk a lot more about these below. Both of these have the same number of carbons and hydrogens. How many do you see?
You should have gotten 18 carbons and 34 hydrogens (one on the OH, none on the carboxyl carbon itself, three on the last carbon, one on each of the carbons in the double bond--that’s 6, then each of the remaining 14 carbons have 2).
Here it is in more explicit form.
Here is a link: screencast covering Bonding Basics.
There is a “table of contents” icon (just 4 horizontal lines at the lower right as you mouse over it). If you click on it, it will list several topics. You can skip to those you want to hear again.
Also, here is a PDF on functional groups that I think you should look over.
Finally, here is a primer on "molecular shorthand" we use when we draw molecules.
Structural Shorthand
When we are writing out a larger molecule, we often use a shorthand notation. So, it is important that you know what they mean. Bonds are line segments, atoms are at the junction or ends of line segments. Double line segments mean double bonds. Beyond that, it’s really simple and based on two rules:
- Carbon is the most common atom in all organic molecules. To save time, I only label things that are NOT carbon. Everywhere else you see a place for an atom (end of line segment) that is not labeled, it is a carbon. Anything else, I will label.
- Carbon will have four bounds around it. If you see fewer than four, the rest are made up by bonds to hydrogens, which I don’t bother to write.

Each end of the line segment is a C, which I don’t write. Each C must have three more hydrogens to fill it out, which I also don’t write.
However, consider this molecule:
 It is a really short fatty acid called “butyric acid.” How many carbons and hydrogens are there.
It is a really short fatty acid called “butyric acid.” How many carbons and hydrogens are there.I’ll wait.
You should have gotten 4 carbon, 8 hydrogen. Here it is explicitly.

The carbonyl carbon has four bonds already (two to the O on top, one to the O to its right, and one to the carbon on the left) and has no room for hydrogens. Carbons in the middle of a chain have two hydrogens and the one on the end has to have 3.
Here’s a much harder one:

We will talk a lot more about these below. Both of these have the same number of carbons and hydrogens. How many do you see?
You should have gotten 18 carbons and 34 hydrogens (one on the OH, none on the carboxyl carbon itself, three on the last carbon, one on each of the carbons in the double bond--that’s 6, then each of the remaining 14 carbons have 2).
Here it is in more explicit form.