Polysaccharides
Polymers of Sugar
or, polysaccharides.
A monomer of sugar, with the empirical formula CH2O, is called a simple sugar. For our purposes, hexoses (6-carbon sugars) including glucose and fructose are most important here. Pentoses such as ribose in RNA and deoxyribose in DNA will be dealt with later.
Sugars have a carbonyl on one carbon and hydroxyls on the others. The carbonyl can be on the end (an aldose such as glucose), or not on the end (A ketose, such as fructose). We often draw them as a linear form. But, in water, they are not.
Sugar Cyclization
Here is the linear form of glucose:
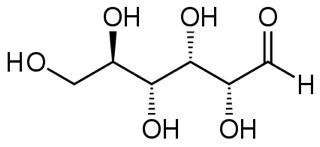
and another view in ball-and-stick model.
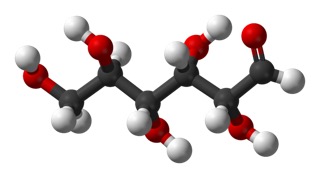
Carbon 1 is on the right, the carbonyl.
The cyclization is just a rearrangement of the atoms in the molecule. No atoms are lost (e.g. water is not released).
The dashed-line bonds are bonds that would go slightly back into the page and the dark wedge-shaped bonds would come forward out of the page, as indicated in the 3-d view.
The chemistry of the interaction, if you care, is that the carbonyl carbon is very electron poor (the brutish oxygen is stealing it’s electrons). The lone pairs on any of the alcohol (OH) group oxygens could in principle initiate a reaction with the carbonyl carbon (alcohols and aldehyde often react...so when you put sugar in solution, it reacts with itself). The most stable ring is formed when the OH on Carbon 5 attacks the carbonyl carbon. As the bonds are exchanging, that oxygen ends up bridging carbon 1 and carbon 5 AND having a hydrogen bonded to it….This intermediate is unstable. Both the oxygen and Carbon 1 are making too many bonds, which cannot stay. So carbon 1 has to lose one bond to what had been the carbonyl oxygen. That leaves the carbon looking good, but we have one oxygen (in the ring) with 1 too many bonds (+1 formal charge) and the other on carbon one with not enough bonds (-1 formal charge). The more stable structures trades the hydrogen off the ring oxygen on (originally from carbon 5) to the former carbonyl carbon on carbon 1.
For those keeping score, this adds one more asymmetric carbon (carbon 1) and one more possible isomer, which we call the alpha and beta form, as we see in either starch or cellulose.
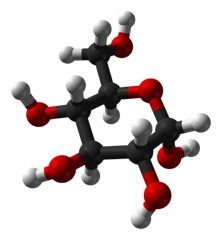
It seems like such a small thing: does the OH on carbon 1 stick straight out of the plane of the ring (called “axial”) as below:
Or does it stick out more in plane with the ring (“equatorial”), as it does in this form:
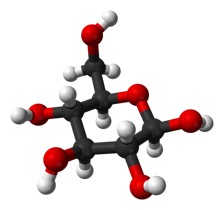
But, it is a big deal. The top form is called alpha and the bottom is called beta.
Polymerization
Polymerization is carried out by an enzyme that joins the joins carbon 1 of one ring with carbon 4 of another. It’s a dehydration synthesis, resulting in the OH from carbon 4 leaving with an H from the hydroxyl on the carbon 1 to form water. The bond can be hydrolyzed by a different enzyme. I said, animals lack the enzymes to deal with the beta form of glucose…But, actually, I was wrong about that, to some extent. We do have a (poor) beta glucosidase…in our tears. It’s called “lysozyme” and it’s part of our defense against bacteria. Also, a sugar similar to glucose (another hexose/aldose with the formula C6H12O6) called galactose pairs in its beta form with glucose to form a disaccharide called Lactose (perhaps you’ve heard of it?). At least as infants, we make a beta galactosidase protein called “lactase.” Most of us don’t make that when we get older and so we have some level of lactose intolerance.
But, in general, we deal poorly with beta forms of glucose in polymers.
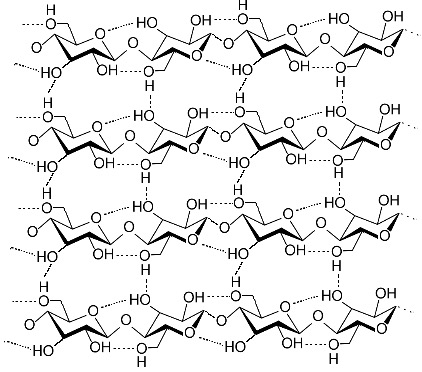
This is a representation of cellulose (also known as “Fiber”) from Wikipedia, the same one we looked at today. It is a main structural component of plants, in what is known as the “cell wall”. Note that there are four VERY short chains linked through the oxygens in a beta 1-4 link. The actual chains would be much longer.
Notice that the oxygen on carbon 1 (right-hand carbon in each ring) is sticking out to the side of the ring. The net result of this is that the sugars alternate orientation. You can see this best by looking at the carbon 6, which is sticking out of the rings, either up or down, alternating.
As a result of this, there are hydrogen bonds both within each chain (from each sugar to the next) and to the chain running along side of it. Seems like a recipe for something fairly strong, but flexible (chains can slide along each other under stress, simply making new hydrogen bonds).
This is a great example of how details of the fine structure explain the behavior of the larger structure. Wood is essentially made of single fibers all cross-linked in many directions that allows at least some sliding, and therefore bending.
Compare it to this, which is starch (we can digest that).
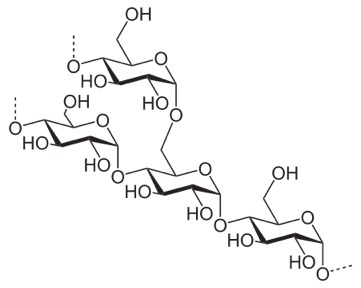
In this case, you see the O off carbon 1 sticking down, out of the plane of the ring. This leads to all the sugars more or less orienting the same way, no great ability for hydrogen-bond cross linking, and sort of a slow, spiraling of the strand.
Key properties of polysaccharides for AP:
Here are some things I would like you to know at this point or very soon.
or, polysaccharides.
A monomer of sugar, with the empirical formula CH2O, is called a simple sugar. For our purposes, hexoses (6-carbon sugars) including glucose and fructose are most important here. Pentoses such as ribose in RNA and deoxyribose in DNA will be dealt with later.
Sugars have a carbonyl on one carbon and hydroxyls on the others. The carbonyl can be on the end (an aldose such as glucose), or not on the end (A ketose, such as fructose). We often draw them as a linear form. But, in water, they are not.
Sugar Cyclization
Here is the linear form of glucose:

and another view in ball-and-stick model.

Carbon 1 is on the right, the carbonyl.
The cyclization is just a rearrangement of the atoms in the molecule. No atoms are lost (e.g. water is not released).
The dashed-line bonds are bonds that would go slightly back into the page and the dark wedge-shaped bonds would come forward out of the page, as indicated in the 3-d view.
The chemistry of the interaction, if you care, is that the carbonyl carbon is very electron poor (the brutish oxygen is stealing it’s electrons). The lone pairs on any of the alcohol (OH) group oxygens could in principle initiate a reaction with the carbonyl carbon (alcohols and aldehyde often react...so when you put sugar in solution, it reacts with itself). The most stable ring is formed when the OH on Carbon 5 attacks the carbonyl carbon. As the bonds are exchanging, that oxygen ends up bridging carbon 1 and carbon 5 AND having a hydrogen bonded to it….This intermediate is unstable. Both the oxygen and Carbon 1 are making too many bonds, which cannot stay. So carbon 1 has to lose one bond to what had been the carbonyl oxygen. That leaves the carbon looking good, but we have one oxygen (in the ring) with 1 too many bonds (+1 formal charge) and the other on carbon one with not enough bonds (-1 formal charge). The more stable structures trades the hydrogen off the ring oxygen on (originally from carbon 5) to the former carbonyl carbon on carbon 1.
For those keeping score, this adds one more asymmetric carbon (carbon 1) and one more possible isomer, which we call the alpha and beta form, as we see in either starch or cellulose.
It seems like such a small thing: does the OH on carbon 1 stick straight out of the plane of the ring (called “axial”) as below:

Or does it stick out more in plane with the ring (“equatorial”), as it does in this form:

But, it is a big deal. The top form is called alpha and the bottom is called beta.
Polymerization
Polymerization is carried out by an enzyme that joins the joins carbon 1 of one ring with carbon 4 of another. It’s a dehydration synthesis, resulting in the OH from carbon 4 leaving with an H from the hydroxyl on the carbon 1 to form water. The bond can be hydrolyzed by a different enzyme. I said, animals lack the enzymes to deal with the beta form of glucose…But, actually, I was wrong about that, to some extent. We do have a (poor) beta glucosidase…in our tears. It’s called “lysozyme” and it’s part of our defense against bacteria. Also, a sugar similar to glucose (another hexose/aldose with the formula C6H12O6) called galactose pairs in its beta form with glucose to form a disaccharide called Lactose (perhaps you’ve heard of it?). At least as infants, we make a beta galactosidase protein called “lactase.” Most of us don’t make that when we get older and so we have some level of lactose intolerance.
But, in general, we deal poorly with beta forms of glucose in polymers.

This is a representation of cellulose (also known as “Fiber”) from Wikipedia, the same one we looked at today. It is a main structural component of plants, in what is known as the “cell wall”. Note that there are four VERY short chains linked through the oxygens in a beta 1-4 link. The actual chains would be much longer.
Notice that the oxygen on carbon 1 (right-hand carbon in each ring) is sticking out to the side of the ring. The net result of this is that the sugars alternate orientation. You can see this best by looking at the carbon 6, which is sticking out of the rings, either up or down, alternating.
As a result of this, there are hydrogen bonds both within each chain (from each sugar to the next) and to the chain running along side of it. Seems like a recipe for something fairly strong, but flexible (chains can slide along each other under stress, simply making new hydrogen bonds).
This is a great example of how details of the fine structure explain the behavior of the larger structure. Wood is essentially made of single fibers all cross-linked in many directions that allows at least some sliding, and therefore bending.
Compare it to this, which is starch (we can digest that).

In this case, you see the O off carbon 1 sticking down, out of the plane of the ring. This leads to all the sugars more or less orienting the same way, no great ability for hydrogen-bond cross linking, and sort of a slow, spiraling of the strand.
Key properties of polysaccharides for AP:
- Cellulose and Starch (and the slightly branched “amylopectin”) are made by plants.
- Glycogen is made in animals (mainly in the liver).
- Starch, pectin and glycogen all use alpha glucose and are mainly storage forms of glucose. Your body, for example, will either make or hydrolyze glycogen to take up or release glucose to your blood.
- Cellulose is the beta form of glucose and used primarily to provide structure.
- Though a strand of polysaccharide is joined from carbon 1 of a glucose to carbon 4 of the next, you can have branching where a long strand will attack carbon 6 (the one outside the ring) with its carbon 1.
- Cellulose has no branching; starch (amylose) has no branching and pectin a little branching. Glycogen is highly branched.
Here are some things I would like you to know at this point or very soon.
- What are polymers? How are monomers assembled into polymers? Explain hydrolysis and dehydration synthesis.
- Identify the number of the carbons in a monossacharide both in linear and ring form.
- What is the difference between an aldose and a ketose sugar?
- What’s the difference between a triose sugar and a trisaccharide? (or hexose versus hexasaccharide).
- How do sugars form the ring structure. Show that no atoms are gained or lost.
- What is wrong with this sentence commonly found in biology texts “cells obtain energy by breaking the bonds in sugar molecules.” ? Students who had me in chemistry may have a leg-up on that one.
- Explain the key differences and similarities between glycogen, starch and cellulose. What makes cellulose a good “structural” polysaccharide? (you just read the answer).