Intro to Thermochem
Intro and principles
All types of energy are interconvertible. The when a reaction takes place that releases energy, such as the burning of paper in air to produce CO2 and H2O, or the reaction of Fe2O3 with Aluminum (thermite), there is energy required to break the bonds initially, and energy released when the new bonds are formed. If the energy of the new bonds is lower on the potential energy hill, there will be a corresponding release of energy to the surroundings. Where does the energy to break the initial bonds come from? Usually it is kinetic energy. It can be kinetic energy from the movement of the molecules themselves. That increases with higher temperature. We can start paper burning by raising the temperature of the paper until the molecules collide with enough energy to break the bonds (which allows the carbon and hydrogen to combine with oxygen in the air to make CO2 and H2O). I can also just use kinetic energy as in the energy I put into moving the two balls that smash into each other. In the second case, the molecules get enough kinetic energy by virtue of riding on the canon balls. It’s all the same.
System and Surrounding
Energy comes in many forms, and can be converted among the forms, but not created or destroyed (this is known as the First Law of Thermodynamics). In order to keep track of energy, we need to define how to follow the changes and define a set of terms.
Here’s how: There is the system, which is the reaction or change at the center of what we are following; there is the rest of the universe, or simply, the surrounding corner of the universe. We are on the outside, watching the system. So, we are the universe, and we report what happens to the system. While there are many ways that we could follow changes in energy (in a car, we can talk about the work done in moving the car, which is the result of release of chemical potential energy in the bonds of gasoline and oxygen being made available when converted to carbon dioxide and water). But, as a practical matter, it’s much easier to follow heat. Heat is abbreviated "q." Under carefully controlled conditions heat (q) and change in enthalpy (ΔH) are just about the same.
Heat is the flow of thermal energy from one body to another. Generally, it is good enough to follow heat as a stand in for enthalpy change. We can never measure the enthalpy of a particular state of matter. But we can measure changes in the enthalpy as matter changes by following how it affects the surroundings. Because of conservation of energy, we assume that whatever heat is gained by the surrounding must have been lost in the potential energy of the bonds in the system. Similarly, if energy is lost by the surroundings (it cools down), that energy has to have been gained somewhere, and it is the form of an increase in chemical potential energy in the system.
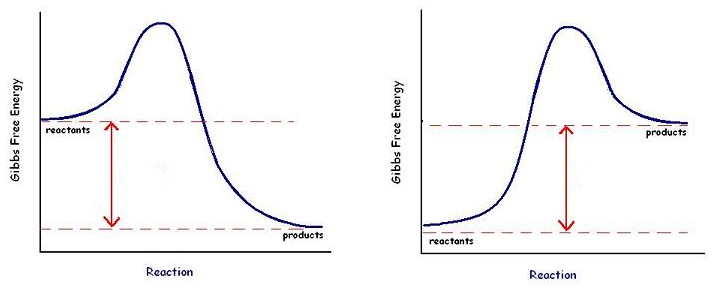
The annoying bit
Okay, so here’s the somewhat odd part: we can only directly measure the changes in the surroundings; however, we like to report what has happened to the system. So, let’s say the surroundings (the water in a calorimeter, or your hand, for example) gain in heat. That must mean the system (the chemical reaction) lost that same amount of energy. So we report the change in enthalpy as negative (the system lost energy...yes, the surroundings gained, but we report what happened to the system). So, the reactants must have higher potential energy than the products, in this case. So this is a negative change in enthalpy from the standpoint of the system. We call this a ‒ΔH. Since energy is coming out of the reaction, we call it “exothermic.” On the other hand, if the surrounding loses energy, that energy must have gone into the system. The system must have gained energy (+ΔH) or endothermic.
Stoichiometry You can use stoichiometry to calculate enthalpy in or out. For an endothermic reaction, it is as though the enthalpy is a reactant that must be put in, while for an exothermic reaction, it is like a product. If it takes 44 KJ to convert one mole of water from liquid to vapor, then converting two moles would be 88KJ. So, this is no harder than any other stoichiometry (no easier, unfortunately). You just figure out how many moles you have of whatever thing you are being asked to burn, or whatever, then multiply it by the number of KJ/mol. How do you know the enthalpy of a conversion? For the most part, you can only do that with calorimetry. This basically runs the reaction or state change either in water or in some other material whose temperature you can measure, called the calorimeter. You run the reaction in a calorimeter and determine the heat flow to the water. If the temperature of the water in the calorimeter goes up, the reaction lost that heat (exothermic reaction). If the water temp goes down, that heat left the water and went into the system (endothermic). If the temperature of the water goes up, heat flowed into the water from the reaction, which must therefore have lost that energy. The reaction is therefore exothermic. For reasons I would rather not get into in detail, if we measure heat under constant pressure, we know that the heat is the same as the enthalpy of the reaction. Just pay attention to the sign. Suppose you run a reaction in a calorimeter and find that 100.0g of water in it increased from 21.2 oC to 32.5 oC. Suppose I run a reaction and determine that reacting 0.5 mole of some chemical with another resulted in the change of temperature above (which will correspond to about 4723 Joules (I'll show you tomorrow how to do that) What would be the ΔH of the reaction per mol of reactant?
Heat and Work
In a calorimeter, as I said, we aim to be totally inefficient. That is, have all the energy come out as heat and do no work (or, a constrained type called PV work).
But, the total energy change of the system that is not in such a calorimeter has to be viewed as the total heat and work.
So, the explosion that goes on in the cylinder of your car's engine heats up the engine block, which heats up the radiator fluid, which passes the heat to the atmosphere. I can quantify that. But also, there is work done moving the car. Or, more directly, there is work done as PV work (remember, PV has units of energy).
Total energy change is heat plus work:
ET=q+w
That's true no matter how you measure the work. However, when using PV work, there is a sign convention you must follow. Since work done by the system on the surroundings will increase the pressure or volume (imagine an explosion in a cylinder), ΔPV (or, at constant pressure, PΔV) has a positive value. But, since it's work done by the system on the surroundings, we want that to have a negative. So, we change the sign. So w= -PΔV and
ET=q-ΔPV or q-PΔV if pressure is constant.
