
Lewis Structures Intro
Bonding and Dots
This is a really good site that deals with this. Highly recommend!
We will cover several areas and do so fairly quickly. In order to help you follow along, I’d like to give you an overview.
Bonding is what we call the phenomenon that leads to atoms combining to form molecules. At some level, our explanations are somewhat invented. I’ll try to be careful in distinguishing between what we know about the phenomenon and our models, which are, at best, approximate explanations.
- The electrons, in particular the valence electrons must be involved.
- As always, energy is key. Forming bonds releases energy (exothermic); breaking bonds requires input energy (endothermic). The laws of thermodynamics say that energy must be conserved and that the tendency will be for energy to distribute. The implication is that stronger bonds occur because the electrons involved must be at a lower potential energy state.
- he model we use now is the Localized Electron model. The assumption is that the two bonded electrons must be mainly in the space between the atoms to maintain the low-energy orbital. That means the two atoms cannot separate without either breaking the bond, or if one of the atoms leave their electron behind.
How do we predict bonding?
So, in the quantum mechanics section, we saw dramatically that an imperfect model such as that proposed by Nils Bohr was extremely useful even though everyone knew it was, essentially, wrong.
If we are going to talk about bonding, obviously, we need to consider valence electrons that are either shared (covalent bonds) or traded (ionic bonds). To describe the electrons in a bond fully would probably require its own sub-discipline of quantum. We won’t do that. We will use a simpler method that is remarkably useful in predicting how representative elements form bonds. It is known as “Lewis Structure," or sometimes "Lewis Dot Structure," because of the way electrons are represented.
We will assume, sensibly enough, that bonds form when by doing so, the elements, via their electrons, come to a lower energy state.
Lewis Dot Structures:
For bookkeeping, we write the atom's symbol with the number of valence electrons around it represented as dots.
The first four (one for Li, 2 for Be, 3 for B and 4 for C) are placed alone at each point of the compass (north, south, east and west) around the atoms symbol. It does not really matter where you put the first 3. When you get to carbon, all four positions are occupied. Then, for the next element, N, you have to put the next electron paired with one of the other 4, leaving you with one pair of electrons and 3 lone electrons. Note that “paired” in this case is not the same as paired in a spin-paired quantum mechanical sense. For O, the 6 electrons are in 4 are in 2 pairs, and two are alone. For F, there is only one lone electron (3 pairs plus one lone makes 7 electrons). Here are some examples depicting atoms.
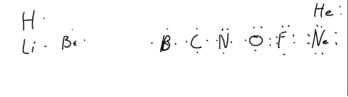
A key point is that, in this model, it is the lone electrons that will determine how it the atom makes bonds. Lone electrons are available for bonding, paired electrons generally are not.
So, Carbon, with 4 lone electrons makes four covalent bonds. Thus, normally, Nitrogen makes 3, oxygen 2 and Fluorine 1. Hydrogen, because there is only one electron and only space for one more, can also only make one bond (there will be cases in which atoms will share either more of fewer electrons than they ideally do).
Based on this, can you make a guess as to why oxygen and hydrogen form primarily H2O and Nitrogen and Hydrogen form NH3?
Simple Covalent bonds
We will be looking in more detail at how bonds form. First, we will look at what happens when non-metals share electrons. The key point is that they share electrons to try to assemble something like the octet of valence electrons found in noble gases (or "duet" in the case of hydrogen, since helium only has two electrons).
In order to do that, they share, such that each atom gets to count the shared electrons as their own. The electrons are therefore in a full valence shell and are therefore at a lower energy state and more stable
To accomplish this, the atoms must be a set distance apart, a distance known as the bond length. We went through how Carbon, Nitrogen, Oxygen and Fluorine form "hydrides." Screen captures are shown below.
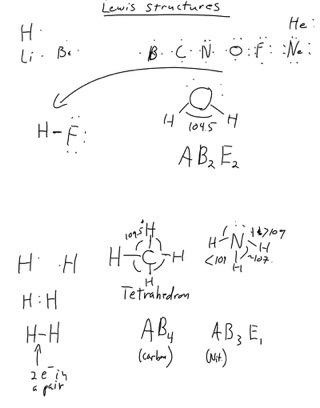
We also talked about how the electron pairs, both bonded and non-bonded, determine the shape and bond angles.
This is based on the idea that electrons pairs on a central atom, whether they are in bonds or lone pairs, will get as far away from each other as possible (Valence Shell Electron Pair Repulsion).
Any molecule that has only two atoms in it (say, HCl) can only be “linear.” If there are two or more atoms attached to the central atom, we have to figure out the atom. We did this with the balloons.
First we count up the number of “Sites.”A “site” is either a bond or a non-bonding pair. In this context, a double or triple bond counts the same as a single.
Sites on a central atom will get as far away from each other as possible. In the short hand we will use, “A” refers to the central atom, “B” refers to bonds (singles, doubles or triples), “E” refers to lone pairs of electrons.
The ones we looked at today were all with some variation of the tetrahedron, which has a bond angle of 109.5 degrees. The more lone pairs, the more that angle is reduced, slightly. In nitrogen, the angle is more like 107, and in water, 104.5.
I've got a pretty simple exercise putting dots on things. It's a bit hard to do in webassign, since you really have to draw stuff. Here is the link to the exercise.
Just answer section for now. For ions, the general rule is that those that form positive ions to get to noble gas configuration is to show that they have lost their valence electrons…therefore, no dots. For negative ions that fill in the valence to get to noble gas configuration, draw all 8 dots.
