Bonding and Naming Compounds
Ionic Bonds:
When a metal bonds with a non metal, they trade electrons. The metal can lose electrons to become more noble-gas like. The nonmetal will gain electrons to become more noble-gas like. Thus each of them becomes an ion (the metal a positive ion, or "cation," and the nonmetal a negative ion, or "anion"). A well-known example is sodium chloride (table salt). Given the opportunity to interact, Sodium will become Na+ and chorine will become "chloride" or Cl-. Now, the result is NOT a Molecule in the true sense of the word. It is an ionic compound. They stick together in a crystal because negatives and positives attract each other. "Salt" is a general term, it turns out. Sodium Chloride is one example of a salt. Traditionally, salts were the product of an acid and a base interacting (As you can make NaCl from NaOH and HCl). But, as a rule, you can refer to ionic compounds as "salts." Why do I say they are not molecules? Well, for one thing, if you look at the crystal in detail, you find that it is just one continuous pattern of positives and negatives packed together. There is no definable "unit." A simpler way to see it is to dissolve an ionic compound in water. The ions break apart from each other and float free as Na+ and Cl- (for example). There is no "sodium chloride molecule" floating in the water. How do I know that? well, one simple way is that the ions in solution will conduct electricity.
While the ions are not new molecules, ions have different properties than their parent atoms. Sodium, as I mentioned, is something you would never want to swallow. You would explode. But, Sodium ions (Na+) are mostly harmless—actually essential for your body in reasonable amounts. Chlorine gas, which comes 'diatomic molecule' (Cl-Cl, or simply Cl2), is a deadly green poisonous gas. Chloride ion (Cl-), again, is mostly harmless and even necessary.
Another important difference
Remember that we described molecules as having new properties unique to that molecule, not found in any of the component elements (see below). Well, Na+, for example, can be found in many different salts, pared with anions that balance out its charge. You can have NaF (sodium fluoride), or NaHCO3 (sodium bicarbonate, also known as "baking soda"). In any ionic compound that has Na+ in it, the properties of that ion are indistinguishable.
So, while the formation of an ion produces a new thing whose properties are different from the atom (Na and Na+ are very different), Na+ has the same properties no matter who ended up with its electron.
Covalent Bonds:
These create a new entity called a molecule that is different from any of the component atoms. Always associate the term "covalent bond" (or polar covalent) with "molecule."
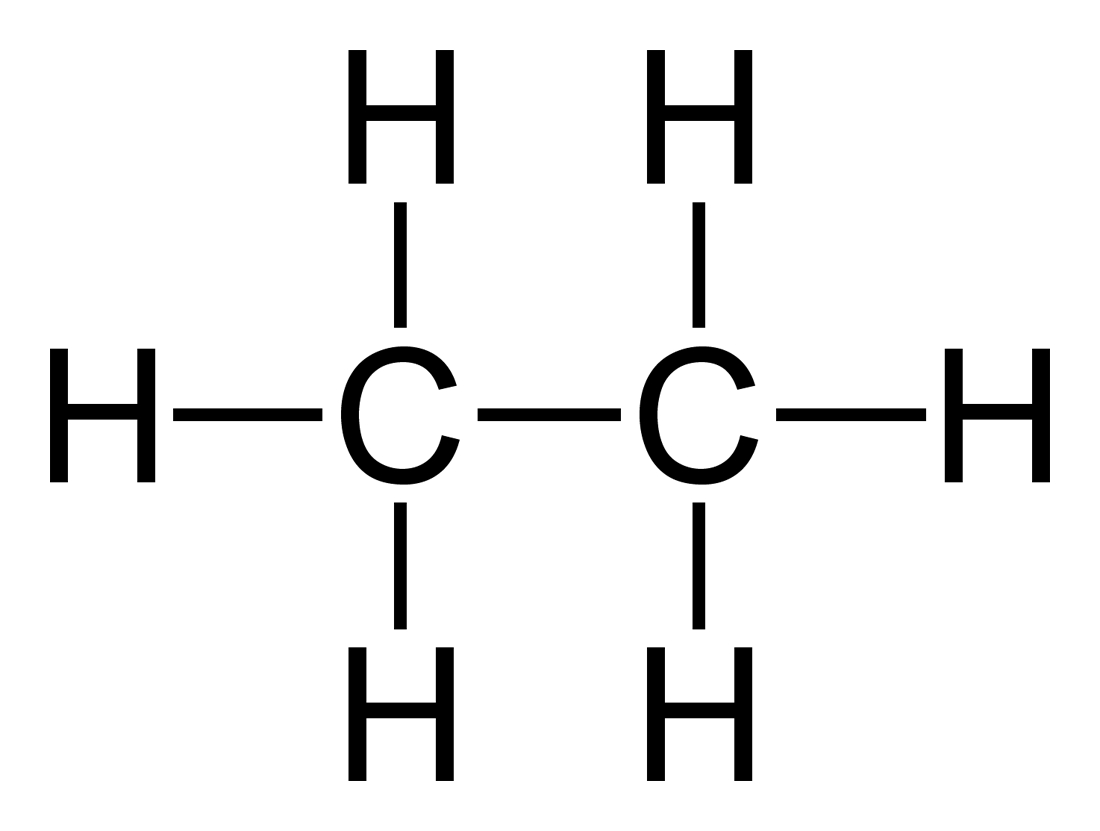
When non-metals bond to non-metals, they share electrons. For example, Carbon and Oxygen each would like to have 10 electrons (like neon) Oxygen needs 2 more, carbon needs 4 more. Each oxygen shares 2 electrons with carbon and gets two back from carbon in the deal, bringing each of them up to 10. The carbon in turn shares four electrons (two with each Oxygen), gets four back in the deal and brings it's number up to 10, like neon. The result, in this case, is carbon dioxide. What about carbon monoxide? Well, it turns out that it is not as stable as carbon dioxide. Note:
• Producing CO releases less heat (remember the yellow flame), which means that CO is not as low in potential energy
• Producing CO does not allow sharing of electrons in a way that gets the atoms to the most stable noble gas configuration.
I'm hoping that the pattern is starting to sink in here. In contrast to NaCl, CO2 is a molecule. It travels as a unit and has its own characteristics. Another example would be sugar. Let's take glucose (C6H12O6). If it dissolves in water, it stays glucose. It does not break up into carbon, hydrogen and oxygen ions. It does not conduct electricity, and in a crystal, one can clearly detect a unit of sugar (we call a molecule).
Polyatomic ions:
These are collections of atoms that form what is essentially a molecule with it's own net charge. The most common of these will have extra electrons and form therefore, polyatomic anions. They are like a molecule in that the atoms comprising the ion are bound to each other in what behave as covalent bonds. They just have an extra electron (or more) that they got from some metal (in the case of polyatomic anions). There are polyatomic cations (positive) that have donated electrons to some non-metal. But, they are less common. Take the base "sodium hydroxide," NaOH. In solution in water, it breaks up into Na+ and OH-. The OH- travels as a unit in solution, so it is a polyatomic ion. Other examples would be Nitrate (you've heard of that) which is NO3-. This is a nitrogen and 3 oxygens bound together with an extra electron, often stolen from potassium or sodium (for potassium nitrate or sodium nitrate respectively).
Formulae
(or formulas, if you prefer)Empirical formula. When considering ionic compounds, remember that the crystal is only a collection of positive and negative ions packed as close together as possible. The only thing that matters is the ratio of the ions, since there are no discreet molecules. In NaCl, It is always 1 sodium ion to 1 chloride, which keeps the overall compound electrically neutral.
In magnesium chloride or calcium chloride, it is always 1 of the metal ions (magnesium or calcium) with two chlorides. Look at the periodic table and see if you can tell why.
Molecular Formulae
Molecules can be reported as having empirical formulae also. It's just the ratio of each atom to the others in the molecule. However, Molecules are more than just atoms stuck around each other in a particular ratio. They are discreet entities in which the arrangement in space creates a structure that is unique to the molecule. These are real, 3-D objects with corners and bends and twists and movable or non-movable parts. So, like other carbohydrates, Glucose has the empirical formula CH2O (hence the term "Carbo-Hydrate" or carbon water). There are lots of molecules that have that ratio of carbon, hydrogen and oxygen (1:2:1). However, the simplest form of this actually has the formula CH2O (usually written H2CO) is formaldehyde, or "embalming fluid." Not something you want to eat at all. On the other hand, C6H12O6 is tasty (actually, that's a bit of a lie…glucose, which has the formula C6H12O6 is not that sweet). So, a molecular formula tells you not only the ratio of atoms, but the exact number of each in the molecule. The molecular formula of a simple sugar will always be a multiple of the empirical (glucose is 6xCH2O).
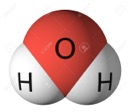
Structural formula: These attempt to tell you not just the number of atoms of each element, but how exactly they are arranged in space. Taking the glucose example again, there are actually several other sugars that have the molecular formula C6H12O6, including glucose and fructose. A structural formula can give varying degrees of information, but shows you how the atoms are bonded together. For example, take water, H2O. That is the molecular formula of water (it also is the empirical formula, since it is the simplest expression of the ratio also). But, how are they bound among each other? Is it H-H-O? No, it's H-O-H. That tells you the way they are aligned, and so is a simple structural formula. However, it gives you the idea that they are in a straight line. In reality, they are bent with the H's about 104 degrees apart. So, I could draw it that way and give you additional structural information. I could also represent the relative size of the atoms, which would be a structural formula with even more information.
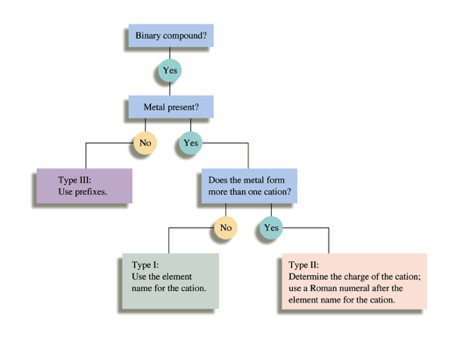
Naming Compounds Flowchart For these, you can use the flow chart from the book:
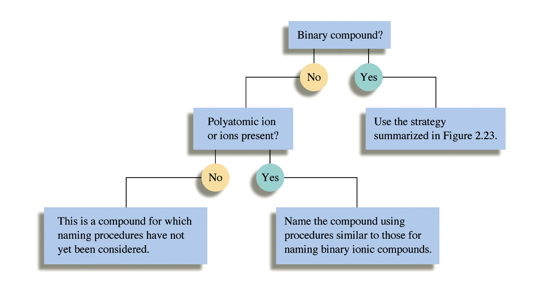
There is also one I put together you may download as a PDF. It's the one I used in the screencast.
To use it, you start at the top and answer the questions, then follow the arrow for the correct answer. The “Guided Practice problems” ask the questions as you would. Note: if you have not yet gotten to naming molecular compounds, don’t worry, we’ll get to it. The required polyatomic ions as well as many other can be found in the book. The mains ones are: Hydroxide (OH-); Ammonium (NH4+ ); Sulfate (SO42-); Nitrate (NO3-); Carbonate (CO32-); Phosphate (PO43-). There will be others.
Guided Practice problems:
- SrCl2. a. Only two elements? yes, so you don't have to worry about polyatomics b. All non metals or metal with a non metal? Sr is a metal, Cl is a non-metal. So, this is an ionic compound. c. Does the metal make only one type of ion? yes, so you don't have to worry about roman numerals. You know this because Sr is in group 2, which always makes a 2+. You also know that Cl always makes a 1- (hence the 1:2 ratio) d. You are ready to name it. It's strontium chloride.
- Fe2O3 a. Only two elements or more than 2. two only b. All non-metals or a mix of metals and non-metals? metal and non-metal therefore ionic c. Does the metal make only one type of ion. No, it is a transition metal, and usually is 2+ or 3+. But, which one is it? Well, Oxygen makes only a 2-. So 3 of them have a total 6-. Since there are only 2 irons, they must be in the 3+ form. It is therefore "iron (III)" remember: the number refers to the charge on the metal, not the number of them! d. Name it: iron (III) oxide.
- NH3 a. Is it only two? yes b. Metals and non-metals or all non-metals? all non metals. Covalent compound c. Use the mono, di, tri prefixes. You could call it mononitrogen trihydride. However, we generally don't use the "mono" for the first element. So, nitrogen trihydride, would be the answer. The common name of this is "Ammonia."
- Cr(SO4)3 a. Only two elements? no, look for polyatomic ions. You have a list of common ones on the back of your periodic table. SO42- is a polyatomic ion (sulfate). The parenthesis with the 3, (SO4)3 means that there are three of them. b. Is it paired with a metal? yes...looks like an ionic compound c. Is the metal one that only makes one type of ion? no. There are several. The table gives you some of them. d. What is the charge on Chromium. Since sulfate is 2-, and there are three of them, the total charge from the anion is 6-. This is paired with only one Cr, so it must be 6+. So, this is "chromium (VI) sulfate."
- What would be the formula of chromium (IV) sulfate? a. Working backwards. Chromium is a metal and its charge is 4+ (because it is chromium IV). b. Sulfate is a polyatomic ion SO42-. So, to cancel out the 4+ of the chromium, there must be two of the sulfates. The formula is Cr(SO4)2
