Protein Structure: Overview
Amino Acids
You should know the basic structure of one, identify the amine, the alpha carbon, the “R” side chain and the carboxyl group (acid). My goal would be for you to be able to recognize most of them. But, the main thing I want you to learn is the categories: which are charged, which are hydrophobic etc. there is a good chart in your book. Given an example, I would like you to be able to identify whether the "R" side chain is hydrophobic or what other important functional groups it has. Example: presented with serine:
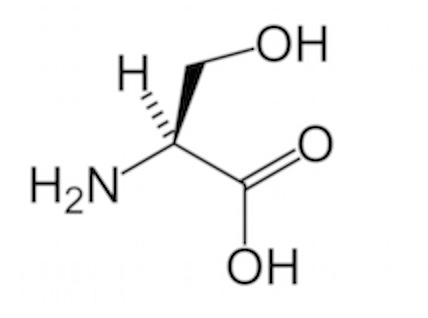
you should be able to spot that the side-chain has an OH (top) on it. You could guess that it is a decent H-bond donor. You might also ask whether that OH is ever chemically attacked by a phosphate (it is, quite often).
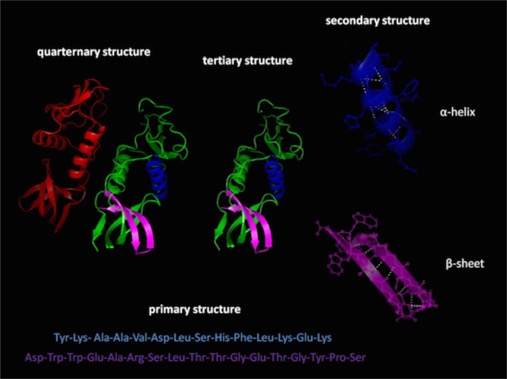
Primary structure
Primary structure is nothing other than the sequence of amino acids that make up the proteins. While I don’t want you to sit and memorize the structures (well, I kind of do, but it’s a lot of memorization), I do want you to get to know the categories as they are identified in the book or at wikipedia. We haven’t gone into that yet. So far we have ignored the “R” groups.
Primary structure just gives you one long chain, like the chain of magnetic beads.
You should understand the chemistry of how amino acids connect to form peptides (dehydration synthesis). At typical peptide will include hundreds of amino acids. Notice that there is an amino terminus and a carboxyl terminus. There are no actual amino acids left after peptide bond formation. They are then called “residues.”
Secondary Structure
Secondary structure is the first step of how the chain of amino acids folds. This does not directly involve the R groups, though, they will have an impact. The secondary structure will be due to hydrogen bonds between the carbonyl oxygen and the amine hydrogen of another residue. Those are part of the backbone.
For our purposes, there are two main secondary structures: ɑ helices and β sheets (often called “beta pleated sheets,” by non biochemists).
Alpha helices will be like the small helices we built with the magnet spheres. There will not be enough space in the center of the helix for other molecules, even water, to fit. They are “right handed” screws. Looking down the spiral from either direction, the spiral runs clockwise away from you.
When we some more complex models later, we will examine three main features:
- The helix is held together by backbone hydrogen bonds between a carbonyl carbon and the amino group of the fourth amino acid along the chain.
- This structure is fairly rigid.
- This repeating structure places all the “R” side chains on the outside (they couldn’t fit in the center anyway). A subtle result of this is that residues 1, 4, 8 etc will be on the same side of the helix.
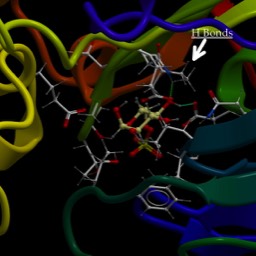
Here is a close up of a helix in the estrogen receptor
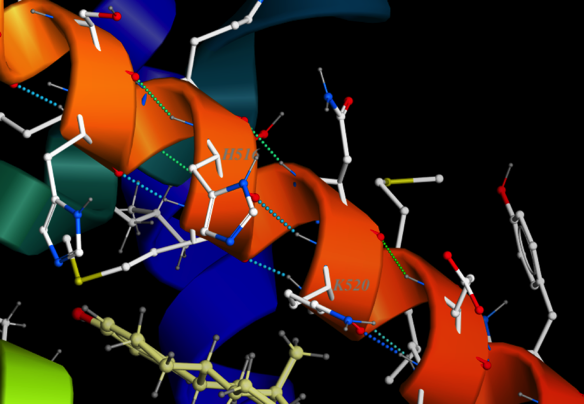
Notice the dotted lines indicating hydrogen bonds between a carbonyl in the backbone (little red sphere for oxygen) and the hydrogen of the amine on the loop in front of it. I have labeled two amino acids. There’s the ringed histadine (you probably are almost able to spot it by now) labeled H516 (that just means it’s the 516th amino acid residue in the primary structure. The one on the same side of the helix exactly one loop further to the right is Lysine 520 (K520). It’s four residues farther along.
Why do some amino acids form helices and some not?
The other common form of secondary structure is called a “beta sheet” or “beta pleated sheet.” (See below) Why do some chains form helices and some form sheets?
Dihedral angles:
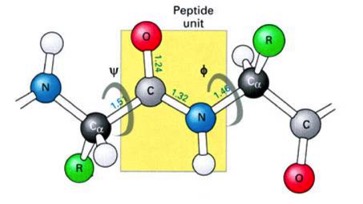 These angles, also known as the “phi/psi” angles denote the rotation around the N-Carbonalpha bond (phi, ϕ) or the rotation around the Carbon alpha-Carbon carbonyl bond (psi, ѱ)
These angles, also known as the “phi/psi” angles denote the rotation around the N-Carbonalpha bond (phi, ϕ) or the rotation around the Carbon alpha-Carbon carbonyl bond (psi, ѱ)
The key thing to know is that, while the atoms are free to spin around the single bonds, there is a preferred dihedral angle. That is generally dictated by the nature of the R side chain. You can force any amino acid (except proline) into the correct angles for an alpha helix. But, some are better at it than others. Alpha helices form because they are strings of amino acids that prefer the correct phi/psi angles. If you have a run of these alpha-helix-preferring residues, they form quickly into the correct structure.
Beta sheets are made up of residues that prefer a slightly different dihedral angle. Again, any amino acid except proline can fit into a beta sheet. But, some residues are better at it.
Beta sheets will be like those flat sheets we made, or, at least the two strands we made, with the magnet spheres. As with the spheres, the sheet can be made either of antiparallel strands or of parallel ones. Also like the spheres, the details of the structure will be different in parallel and antiparallel sheets. However, the wonderful beads breakdown as a nearly perfect model at this point.
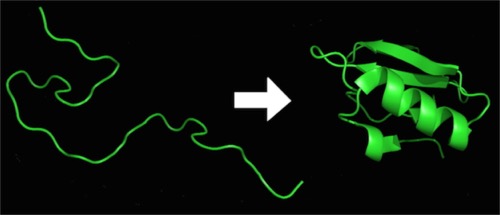
Folding of proteins and structure:
Recap: proteins are made as one long strand of hundreds of amino acid residues (the largest know has over 33,000 residues). Given that the average mass of an amino acid is 110 Daltons, a “typical” protein might have a mass of 50,000-60,000 daltons and a really big one in the several million Daltons, We actually use “KiloDalton,” 1000 Daltons, as the unit of choice. So, 50,000 Dalton would be 50KD.
They are in a line and the sequence of residues is known as the primary structure. There is an amino-terminus and a carboxyl terminus. But, there are no actual amino acids left, since water was removed when the peptide bond was formed between each residue.
Secondary structure, for A.P. bio, is simplified to be alpha helices and beta sheets. There are more subtle things I want you to know. First and foremost, I want you to know that there are more subtle things. You might encounter a 3/10 helix or a pi helix (we may build them). You know that beta sheets can fold into a barrel, like the one we folded.
My wife recently solved one that is a “beta propeller.” It’s a cool variant on a beta sheet.
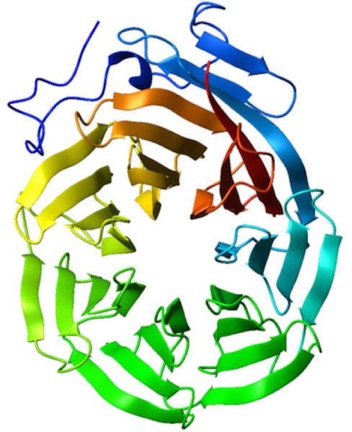
Secondary structure is mediated by the backbone carbonyls interacting with backbone amines.
The last thing about secondary structure I mentioned was that an alpha helix is easy to make with one side hydrophobic and the other hydrophilic by incorporating residues with hydrophobic side-chains in every fourth position. This is called an amphipathic helix. Beta sheet have side-chains that alternate Up/down along each strand.
Structures with lots of alpha helices tend to be soluble in water. Beta sheets, not so much.
Remember that, while the “R” groups or side-chains of amino acids are not directly involved in secondary structure, they influence the dihedral angles and thereby indirectly determine what will form.
Tertiary structure:
This is the first aspect of structure that is mediated directly by the “R groups” or side-chains. The side chains influence the preferred dihedral angles and therefore influence secondary structure. But tertiary structure results from how helices and sheets come together in a 3-D shape.
Tertiary structure may be mediated by hydrophobic interactions, leaving the hydrophilic faces on the surface if the protein is found in water. But, there may also be “salt bridges” formed by interactions between negatively charged (Acid) side chains on one section with a positive (Basic) side-chain on another.
There also are covalent interactions, where to Sulfhydryl-containing side chains form a disulfide link. Two cysteine residues that may be very far apart in the primary structure may fold so that the structures in which they reside come close together. They can then form a covalent link:
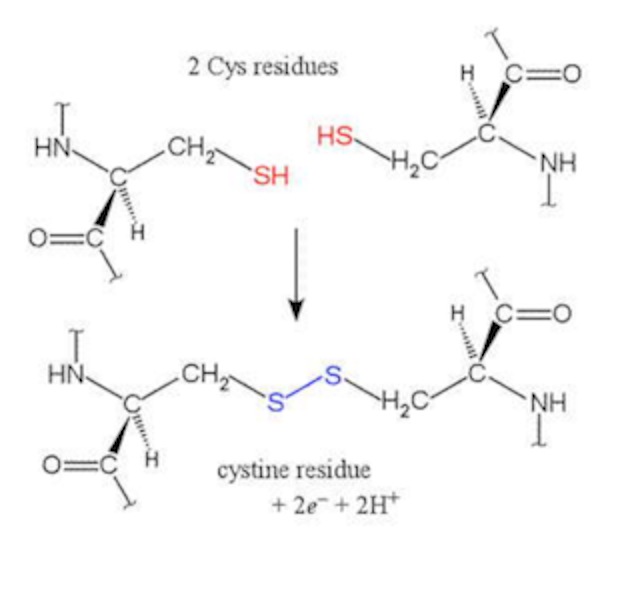
This is an oxidation, as two electrons are removed. It looks like you would get hydrogen gas out. But, usually, the electrons go somewhere else.
Quaternary Structure:
This is when two or more folded polypeptide chains interact to form a larger structure. These are mediated by the same interaction as found in tertiary structure.
`
MathJax Font

Made in RapidWeaver




 These angles, also known as the “phi/psi” angles denote the rotation around the N-Carbonalpha bond (phi, ϕ) or the rotation around the Carbon alpha-Carbon carbonyl bond (psi, ѱ)
These angles, also known as the “phi/psi” angles denote the rotation around the N-Carbonalpha bond (phi, ϕ) or the rotation around the Carbon alpha-Carbon carbonyl bond (psi, ѱ)